Freezing
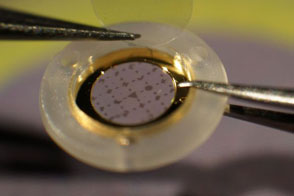
The aim of cryo-EM is visualising the native structure of a biological specimens without using chemical fixatives, contrasting agents or stains. Samples surrounded by aqueous solution, which have grown or applied on grids, are first blotted with filter paper until a thin film remains. Subsequently, the samples are rapidly frozen in a process termed “vitrification”. This fast freezing process embeds the sample into a glass-like amorphous ice and prevents that damaging ice crystals can form which would otherwise distort the cellular ultrastructure. Cells, bacteria, viruses as well as single particles can be vitrified by "plunging" the grids into liquid ethane (secondary cryogen). This process is called "plunge freezing". Tissue parts or small organoids are too thick and cannot be vitrified fast enough this way. For these samples, "high-pressure freezing" is available. Using liquid nitrogen and a pressure of over 2.200bar, samples of up to 200µm can be frozen without ice crystal formation. Cryofixed samples can be stored in special grid boxes (cryo-pucks) until further processing.